The Question of how Life began on Earth remains one of the most profound and elusive Mysteries in Science. For Decades, Theories have ranged from Primordial Soups energized by Lightning (the famed Miller-Urey Experiments) to deep-sea Hydrothermal Vents fostering complex Chemistry. Yet, the deep Complexities and unyielding Challenges of Abiogenesis — the transition from Non-living Chemistry to Living Cells — remain completely unresolved.
Media Hype hops “Man Made Frogs” around in the Laboratories…
Reading the Headlines in Newspapers and Popular Scientific Magazines over the past couple of Decades have led many People, even well-educated People, to believe that Scientists have not only created living Cells in the Laboratory, but even higher Lifeforms such as Reptiles. Nothing could be further from the Truth! It is all just one big Hype, verging on Fraud.
The Death Blow
In a revealing and sobering Conversation, Dr. James Tour and Ocean Chemist Ed Peltzer, a protégé of Miller-Urey pioneer Stanley Miller and Meteorite Amino Acid specialist Jeffrey Bada (Video at the end of this Article), offer a critical re-examination of long-held Assumptions about Life’s Chemical Origins. Their Insights caution against simplistic Interpretations of Experimental Data and the over-optimism that has clouded the Field.
The Mirage of “Plausible” Chemistry: Constrained Conditions Are Not Natural
A central Theme emerging from Peltzer’s Work is the necessity of Constrained Chemistry — the idea that Starting Materials must be pure, Stereochemically uniform, and specific for Life’s Molecular Building Blocks to form.
Meteorites have often been cited as natural Providers of Life’s Precursors. Yet, Peltzer explains reality is far from simple. Meteorites deliver a vast and Chaotic Mixture of millions of different Compounds, devoid of the Stereochemical purity required for biologically relevant Molecules. Without such Constraints, Chemistry devolves into a tangle of competing, cross-reacting Mixtures that are not “usable” by Life’s standards.
Peltzer emphatically states:
“You need Constrained Chemistry. Meteorites do not provide the Stereo-controlled Compounds you need, and the overwhelming Mixture of millions of Compounds makes them useless for the Origin of Life. People think just because something is there, it can be used. No, it cannot.”
This view resonates with others, including Dr. Richard Bliss, who notes,
“The step from simple Organic Molecules to Self-replicating Systems is vast. Laboratory results like Miller-Urey do not replicate Natural Conditions accurately, and the Mixtures formed are chemically impure and unstable.”
Peltzer and Bliss warn that Laboratory Separation using Chromatography and Mass Spectrometry — tools not present in any primal world — highlight how far removed crude Mixtures are from usable Building Blocks.
The Miller-Urey Experiment Revisited: Amino Acids Amid a Chemical Quagmire
Stanley Miller’s landmark Experiment in the 1950s demonstrated that Amino Acids, one of Life’s Building Blocks that are needed to form Proteins, such as the important group of Proteins known as Enzymes, could form from simple Gases subjected to electrical Sparks. This breakthrough galvanized the Field. However, Peltzer explains the reality is messier than simple Amino Acid formation.
Miller’s setup generated a Complex Mixture including Non-Biological Amino Acids, Amines, Carboxylic Acids, and reactive Aldehydes. These components undergo Maillard Reactions — complex, messy Chemical Reactions producing tar-like, dark-colored byproducts that destroy Amino Acids and other valuable Compounds.
Dr. Brian Miller, a Physicist engaged in the Origin-of-Life debate, concurs:
“Origin of Life Experiments often produce Racemic Mixtures and complex Chemical Messes instead of the selective, pure Compounds necessary for Life’s Molecules.”
Dr. Tour frequently critiques the overly optimistic narrative surrounding the Miller-Urey Experiment:
“Those who think Scientists understand the issues of Prebiotic Chemistry are wholly misinformed. Nobody understands them. Maybe one day we will.”
— Dr. James Tour [Dr. Wile Blog, 2023]
Echoing this outlook, Dr. Stephen Meyer clarifies:
“Producing basic Molecules in a Laboratory is far removed from creating Life itself. The challenge is not just Chemistry but assembling Information-Rich Systems capable of Reproduction and Metabolism.”
— Hoover Institution, 2024
Hydrothermal Vents: Not the Cradle of Life Many Imagine
Hydrothermal Vents deep in the Ocean have been touted as fertile Environments for Life’s Origins due to their rich Chemistry and Energy flows. Yet, Peltzer’s firsthand Research reveals a harsh, inhospitable Reality.
The extreme Heat (often exceeding 300°C) and high Pressures at these Vents accelerate Racemization and decomposition of Amino Acids faster than they could polymerize into Proteins. Complex Organic Molecules like Lipids and Nucleotides are quickly destroyed in such Conditions.
Dr. Harold B. White, a Physicist commenting on Abiogenesis, affirms:
“The creation of Life from Non-life under Natural Conditions confronts overwhelming Chemical Complexity and Thermodynamic Obstacles. The Laboratory Conditions that yield Amino Acids are highly controlled and not representative of Early Earth.”
Dr. James Tour reinforces this:
“You don’t get Polymerization spontaneously. The Free Energy on all these couplings is Positive, and Polymers would rather be two Molecules than one.”
— Dr. James Tour [Maybe God Podcast, 2024]
The Sugar and Lipid Conundrums: Complexity Compounds the Problem
Life’s key Molecular Families — Amino Acids, Lipids, Sugars, and Nucleotides — all present unique Challenges that Origin-of-Life Chemistry must address.
Sugars, essential for DNA, RNA, and Energy Metabolism, are notoriously difficult to synthesize non-enzymatically. The widely proposed Formose Reaction to produce Sugars yields billions of different Compounds in a chaotic Mixture, most of which cannot be used biologically.
Lipids, necessary for forming Cell Membranes, contain Ester Bonds that hydrolyze easily and require stereochemical precision to form stable Bilayers.
Enzymes from living Organisms solve these Problems, but without Life, there are no Enzymes to catalyze Reactions with high specificity. This Complexity underscores Dr. Tour’s caution that assumptions about spontaneous Molecular Assembly in harsh Environments are fraught.
The Complexity of Life Increasingly Out of Reach
Since the Miller-Urey Experiment, Biology and Biochemistry have advanced dramatically, revealing the staggering Complexity inside even the simplest Cells. Non-covalent Interactions and Molecular Configurations number in astronomically high Combinations, far beyond early simplistic Models.
Astonishing Discoveries like Chiral-Induced Spin Selectivity (CISS) suggest Life required precise Molecular Chirality and Control from the outset, not something that could develop gradually from random Mixtures.
Indeed, Scientists now realize that the Target for Abiogenesis has moved ever farther away with new Discoveries, not closer. Dr. Tour sums it up bluntly:
“The Complexity of even the simplest Cell is beyond what many appreciate. Every year, new Information makes the Origin-of-Life Problem harder, not easier.”
— Dr. James Tour, Science and Culture Magazine, 2025
Humpty Dumpty Can’t Be Put Back Together
Even if all these Synthetic struggles weren’t enough, Peltzer and Tour emphasize that deconstructing and reconstructing a living Cell with all its Polymers, Codes, and complex Molecular Machinery remains beyond current Scientific Capabilities.
Even if Scientists had all of Life’s constituent Molecules in pure form, placing them together to form a functioning living Cell is an entirely different Problem — one we currently have no Blueprint or Method to achieve.
Final Thoughts
The message from experienced Chemists and Scientists like Ed Peltzer, Stephen Meyer, Brian Miller, Harold White, Richard Bliss, and James Tour is clear and humbling:
- The Origins of Life are far more chemically Complex and constrained than many Models admit.
- The idea that simple, random, Natural Processes in plausible Early Earth Environments could produce Life’s Building Blocks without Guided Intervention is increasingly doubtful.
- Existing Experimental Models like Miller-Urey and Theories about Hydrothermal Vents encounter severe Chemical Obstacles in producing pure, stable, functional Biomolecules.
- Advances in Biochemistry reveal even greater Complexity that must be accounted for in a coherent Theory of Life’s Emergence.
This does not imply the Question is unsolvable, but it underscores the need for honest, rigorous Chemistry-based Assessments and a willingness to recognize the Limitations of Current Paradigms.
Dr. James Tour and Dr. Edward T. Peltzer’s talk: “Death Blow to Origin of Life Research, Interviewing Chemist on Hydrothermal Vents & Miller Urey“
Learn More:
- Interview with Ed Peltzer and Dr. James Tour: Death Blow to Origin of Life Research
[YouTube] https://www.youtube.com/watch?v=_LWqGVgr9J0 [1] - Dr. James Tour on how little we know about the origin of life
[Dr. Wile Blog] https://blog.drwile.com/dr-james-tour-tells-us-how-little-we-know-about-the-origin-of-life/ [2] - Shocking Truth About Life’s Origins (Podcast Episode)
[Maybe God Podcast](https://www.maybegodpod.com/maybe-god-episode-113-james-tour [3] - Beyond Evolution: Unraveling the Origins of Life with Stephen Meyer and James Tour
[Hoover Institution](https://www.hoover.org/research/beyond-evolution-unraveling-origins-life-stephen-meyer-and-james-tour [4] - Stanley Miller and Harold Urey’s Original 1959 Paper
Miller, S. L., & Urey, H. C. (1959). Organic Compound Synthesis on the Primitive Earth. Science, 130(3370), 245–251. - Critical Discussions of Abiogenesis Chemistry
Various academic critiques summarizing chemical and thermodynamic challenges in origin-of-life science.
Visualizing the Chemistry: Key Diagrams to Understand the Origin-of-Life Challenges
Miller-Urey Experimental Setup and Results
Description: The classic sealed system with water, methane, ammonia, and hydrogen gases, energized by electrical sparks producing a mixture of amino acids and other organics amid a complex chemical soup. Important to note the complex mixtures formed, not pure biochemicals.
The Maillard Reaction mixes into this and messes up the reagents leading to Complex Tars. Sugars and Amino Acids react in non-living chemistry to form polymeric, colored Compounds (tars) that degrade useful Organic Molecules, which is a Major Hurdle in Prebiotic Chemistry.
Extreme Temperatures and Chemical Conditions in Deep-sea Hydrothermal Vents lead to Organic Molecules rapidly decomposing and carbonization, undermining their stability.
Chirality and Racemization in Amino Acids
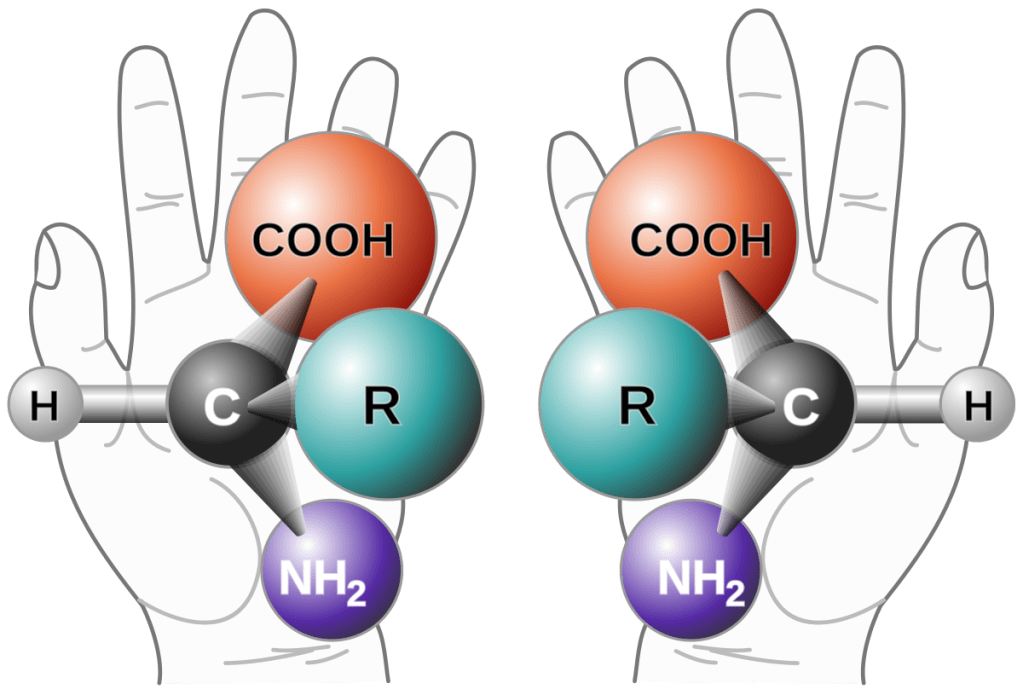
Description: Illustrates the two Mirror-image Forms of Amino Acids, highlighting the Problem that Prebiotic Synthesis yields racemic Mixtures whereas Living Systems use only One Chiral Form.




Leave a comment